Mutations in the Pandemic Influenza Virus
Since seasonal influenza virus is constantly mutating through antigenic shift, it is necessary to develop new vaccines every year, but it could take over 6 months to develop a new vaccine that matches the new virus. In a pandemic setting, many people suffer from infection in the meantime. Therefore, the development of a universal influenza virus vaccine is urgent.
On the 7th, a consortium consisting of Mount Sinai Icahn Medical School, PATH, and etc. in the United States published a paper containing the results of successful phase 1 clinical trial on the universal influenza virus vaccine candidates in the journal ‘Nature Medicine’.
‘The Chimeric HA Vaccine’ Targeting Hemagglutinin Stalk
Conventional vaccines induce neutralizing antibodies that bind to the head domain of hemagglutinin (HA), the surface protein of the influenza virus. The HA head easily binds to antibodies, but has the disadvantage of frequent mutations.
On the other hand, the collaborative research team targeted the stalk domain of HA for the development of a new universal influenza vaccine. The HA stalk is less apt to vary and is shared by various variant virus. Therefore, the antibodies that bind to the HA stalk may be effective against various influenza virus.
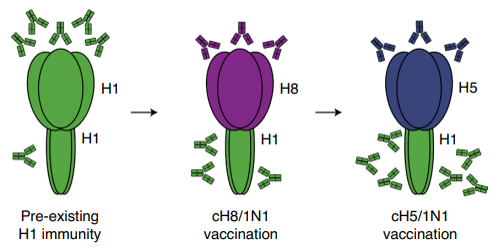
The inactivated ‘Chimeric HA’ vaccine developed by the research team is the combination of the HA head of avian influenza virus and the HA stalk of human influenza virus. Since the human immune system produces a stronger immune response to viruses that have invaded in the past, the immune response to the HA stalk is stronger than the HA head of the avian influenza virus, which is unfamiliar to the human body.
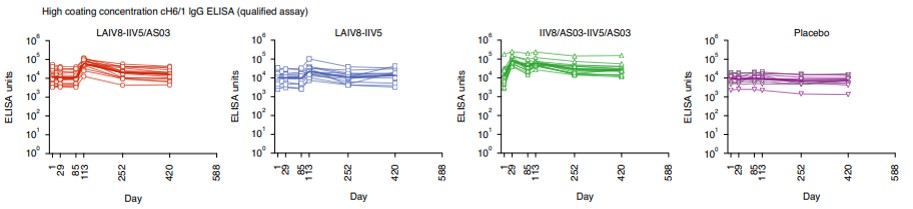
The team’s phase 1 clinical trial evaluated the safety and immunogenicity of the vaccine in 65 participants in the U.S. As a result, only one participant had side effects, and more anti-stalk antibodies were made in the vaccinated participants than in the placebo group. Moreover, a single shot induced remarkably high anti-stalk antibody titers, and a strong immune response endured for at least 18 months following vaccination. The group vaccinated with AS03 adjuvanted prime and boost showed the strongest antibody response. The study is a serious effort to test the stalk antibody hypothesis and an important first step to the development of universal influenza vaccine.
iNtRON’s PHAGERUS® Technology
Since iNtRON is well aware of the market’s “high unmet needs” for universal influenza vaccine, we are striving to develop a universal influenza vaccine as the primary pipeline for our anti-virus platform, PHAGERUS® technology. In addition, by applying the universal vaccine concept to PHAGERUS® technology, we plan to sequentially expand the scope of development to the current world-threatening COVID-19, seasonal influenza, avian influenza, and G4 virus vaccines. iNtRON will evolve into a ‘global R&BD group’ with a variety of new platform technologies through itLysin® technology targeting bacteria and PHAGERUS® technology targeting viruses.
it is iNtRON.

Source :
A chimeric hemagglutinin-based universal influenza virus vaccine approach induces broad and long-lasting immunity in a randomized, placebo-controlled phase I trial
Nachbagauer, R., Feser, J., Naficy, A. et al.
A chimeric hemagglutinin-based universal influenza virus vaccine approach induces broad and long-lasting immunity in a randomized, placebo-controlled phase I trial. Nat Med (2020). https://doi.org/10.1038/s41591-020-1118-7
