iNtRON is continuously expanding its R&BD platform based on Innovative Innovation, and has made new technological changes periodically. Through the expansion of this R&BD Platform Technology, we will identify new MoAs and develop various first-in-class drugs.
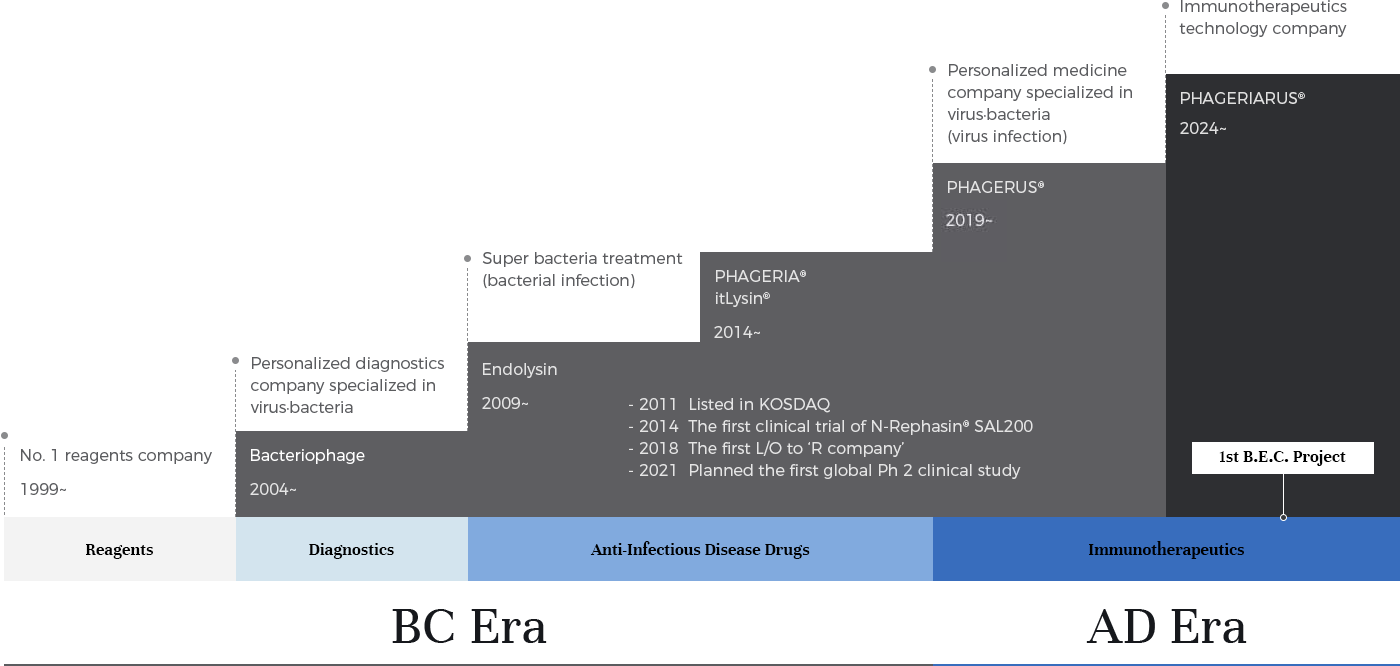
iNtRON, which started as a reagent company in 1999, has expanded its business to molecular diagnostics. Along with this, we focus on full-fledged investments in bacteriophage and endolysin technologies. We continue to do our best to secure core technologies to the extent of holding more than 400 related patents.
In particular, we focus on the potential for success of endolysin as a super-bacteria treatment. In 2014, we started the world's first human clinical trial for SAL200, an endolysin-based leading-pipeline and MRSA super-bacteria treatment. During this clinical trial, we signed a technology transfer contract (L/O) for the SAL200 treatment with "R company" in Switzerland in 2018, and we successfully transitioned from a reagent and diagnostic company to a pharmaceutical company. Since then, we have launched a number of related drug development programs.
In 2019, we advanced PHAGERUS® platform technology, extending the application of bacteriophages from bacterial diseases to antiviral vaccines and therapeutics. In particular, we further strengthened our virus vaccine and therapeutic technologies by securing differentiated mRNA mucosal vaccine platform technology by grafting mRNA-related technologies.
iNtRON defines the aforementioned technologies that focus on bacterial and viral infectious diseases as “BC Era (Before Anti-Cancer Immunotherapeutics, or Before Concept)”. We also define technologies that focus on Immune & Immunotherapeutics beyond infectious diseases as “AD Era (After Anti-Infectious Disease Drugs, or After New Definition/Doctrine)”, and we are accelerating the development of related technologies and creation of pipelines.
The first project of PHAGERIARUS®, a key technology in the AD era and immune & immunotherapeutics, is called B.E.C. Project and targets cancer. Through this project, we will expand our PHAGERIARUS® platform technology and ultimately develop multiple immune regulators as immunotherapeutics.
1999~
itLysin® 2014~
(virus infection)
mRNA 2019~
- 2014 The first clinical trial of N-Rephasin® SAL200
- 2018 The first L/O to ‘R company’
- 2021 Planned the first global Ph 2 clinical study
