[iNtRON] Successful Development of Dengue Fever Diagnostic Kit and Moves to Clinical Trials
▶Plans clinical trials Korea University Hospital and Hanoi University Hospital in Vietnam
▶Expects the product approval in 2024
We would like to announce that the company has successfully developed a specialized immunochromatography-based dengue fever diagnostic kit in the DR part and are now proceeding with clinical trials.
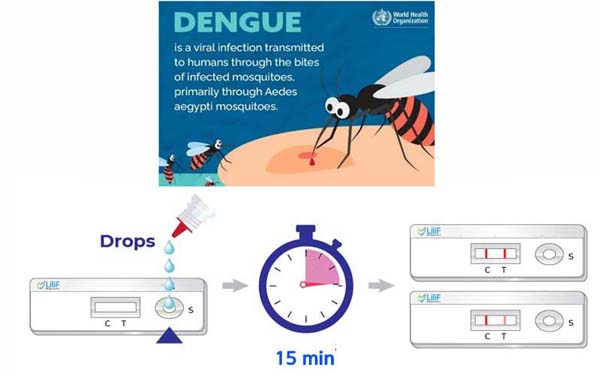
The newly developed dengue fever diagnostic kit can utilize blood samples of all conditions (whole blood, plasma, and serum) without specific restrictions. There are two types of products that can rapidly detect dengue virus antigens and antibodies within 15 minutes on-site. In internal analytical tests conducted after the completion of development, it was confirmed that the product performance is superior compared to other competing products.
We are currently seeking a regulatory approval from the Ministry of Food and Drug Safety (K-FDA) for the two diagnostic kits. To receive an approval, clinical trials are planned at Korea University Hospital and Hanoi University Hospital in Vietnam. And IRB approval for the recent clinical trials has been obtained at each institution. We plan to commence clinical trials in the first half of next year and apply for the product approval from the Ministry of Food and Drug Safety.
Notably, while most existing dengue fever diagnostic kits have only conducted small-scale clinical trials for the export permit purposes, we are planning formal clinical trials for the official product approval. Despite being a latecomer in the field, we aim to differentiate itself by prioritizing the assurance of product quality and plans to secure brand competitiveness by the official approval from the Ministry of Food and Drug Safety.
Dengue fever is an infectious disease transmitted by mosquitoes, specifically the Aedes aegypti and Aedes albopicus mosquitoes with the dengue virus. According to the World Health Organization (WHO), approximately 390 million cases of the infection occur worldwide each year. The incidence of dengue fever is continuously increasing, along with other infectious diseases such as malaria, due to factors like climate change.
SEOL, Jae Goo Ph.D., senior executive director of DR Biz department, stated, "Dengue fever is a globally spreading infectious disease with a large market size, and rapid diagnosis on-site is crucial." He expressed “We have high expectations for the newly developed product, because it is expected to significantly contribute to securing the competitive edge of LiliF® diagnostic brand.
Thank you.
it is iNtRON.
